The TU patented a new cheap base material for semi-organic perovskite solar cells. “This is going to be consequential.”
The new material has an impossible name (EDOT-OMeTPA) but it carries the promise of cheap printable solar cells of just 0,2 microns thick with an efficiency of 11 percent.
“Our synthesis brings commercial applications within reach”, says Professor Theo Dingemans who develops polymers and new materials at the Faculty of Aeronautical Engineering. “It’s up to designers to find applications for it. Wearable electronics could be one, you could print a solar panel on your tent or even on wood panels.”
So what is this magic material? Well, it’s the replacement for another material with an equally strange name (Spiro-OMeTAD) but for a price that is ‘an order of magnitude’ lower. Dingemans and his former Ph.D. student Dr. Michiel Petrus have published(*) a negligible price for their base material.
The material that Dingemans and his team developed forms a hole-transporting layer that functions very well with semi-organic perovskite solar cells. Synthetic perovskites (named after the Russian mineralogist L.A. Perovski) are crystallised metal-organic materials that are cheap to produce and easy to manufacture. They offer many interesting and intriguing properties, one of which is photovoltaics.
Until recently however, perovskite solar cells had very moderate efficiencies of just a few percent. That changed when Henry Snaith and Mike Lee at the University of Oxford reported a perovskite solar cell with an efficiency of over 10 % in 2012. Later, record efficiencies of 20 % were reported.
The disadvantage of the material they’d used was the difficult and expensive synthesis that required rare metals as catalysts and extensive purification.
When Dingemans looked at the structural formula, he was reminded of Schiff-bases, a class of materials invented by the 19th-century German chemist Hugo Schiff. But in contrast to the material used by the Oxford group, Schiff-bases are easily made from cheap materials with high efficiencies and very little waste. “Any first-year chemistry student can do it”, says Dingemans, who provided the recipe for his ‘one pot approach’ in his publication.
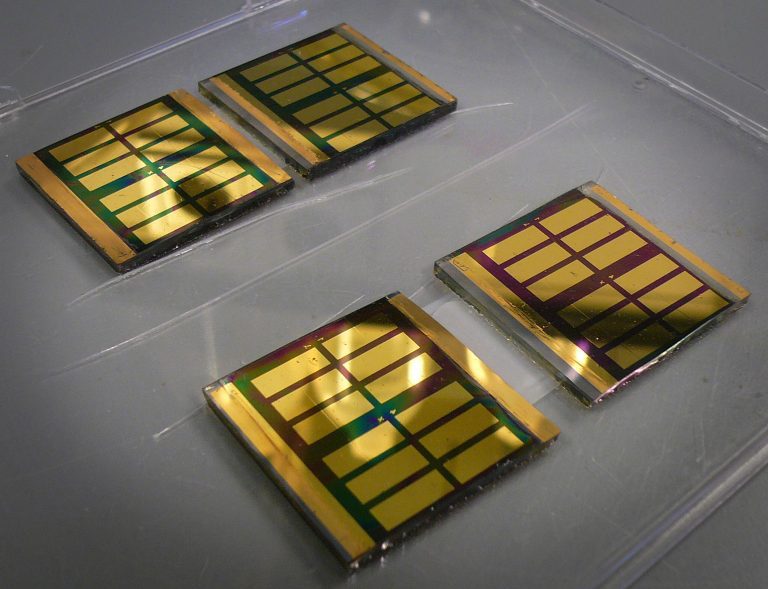
The perovskite solar cells with the Schiff-base material have shown an efficiency of 11 %, which makes them a serious alternative for silicon solar cells (with efficiencies between 15 and 35 percent). “Perovskites have long been regarded as the ugly duck of photovoltaics. But the current technology is going to be consequential. These cells will be applied”, said Dingemans.
Work remains to be done on packaging (perovskite cells are not waterproof) and on stability (improving the lifetime over 1.000 hours). Replacing the lead ions with tin would be an environmental improvement. Start-ups are busy designing industrial production lines.
Dingemans expects printable perovskite solar cells to pop up in disposable applications first. Such as wearable solar cells and solar canvasses. In the longer term, he’d like to make structural polymers, such as used in airplanes and satellites, energy-producing. After eight years of development, Dingemans is convinced that perovskite PV is now on its way from the lab to applications.
(*) M.L. Petrus, T. Bein, T.J. Dingemans, P. Docampo: A low cost azomethine-based hole transporting material for perovskite photovoltaics, Journal of Materials Chemistry A, 11 May 2015, doi: 10.1039/ct5a03046c


Comments are closed.