The government is looking for techniques for producing new fuels from CO2, light and water. Late last year, the Foundation for Fundamental Research on Matter (FOM), the Netherlands Organisation for Scientific Research (NWO) and Shell allocated € 5 million to this purpose.
One o f the seven proposals to be funded was from TU Delft.
Tumult in the town of Doodstil, more and stronger earthquakes in Groningen and a majority in the Netherlands House of Representatives for reducing the gas-exploration activities in the area – these developments signal the end of an era in which natural gas was an obvious source of energy and income for the state (€ 12 billion in 2013). Fifty years ago, the Netherlands could rest comfortably on the prosperity brought by natural gas. Today, however, this comfortable confidence is coming to an end. In the meantime, the Netherlands has acquired a unique infrastructure of gas pipelines, and it would be a shame not to use it. What should we do now? Import gas from Russia? That is as inevitable as it is unwise. Just ask the inhabitants of Ukraine about the difficulties associated with dependence on Russian natural gas. In the long run, however, do we have any alternatives?
The research programme entitled ‘CO2-neutral fuels’, which was launched in the spring of 2013 by FOM, NWO and Shell, has made € 5 million available for the clean production of CO2-neutral fuels from water and carbon dioxide. The objective is illustrated in a cheerful video: electricity from solar panels supplies a factory in which water and carbon dioxide (CO2) are converted into methane (CH4). The gas flows through pipes to reach the farthest corners of our country. As natural gas – in this case, more specifically, solar gas – is burned, carbon dioxide and water are released, thereby closing the circle. In this way, we can live happily ever after.
Storage
Storage is another matter to consider with regard to the advance of solar energy, according to Dr Arno Smets (EEMCS). Although solar energy currently accounts for only a small share (1%), what will happen when this share reaches 10% or 20%? What are we to do with all of this solar energy on a warm summer day? “Storage will become a bottleneck”, predicts Smets. “We have to be able to dump energy through a chemical conversion.” One way to do this would be to generate hydrogen through electrolysis (i.e. using electricity to split H2O into H2 and O2) and converting it into methane (CH4) using CO2. Unlike hydrogen, methane lends itself well to storage, if necessary in the gas fields of Groningen, which would then be empty.
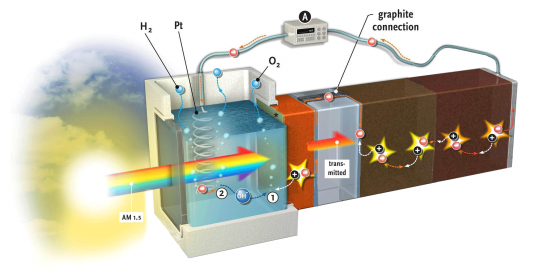
A video on the research conducted by Lihao Han (EEMCS) and Fatwa Abdi (Applied Sciences) on a solar cell that produces hydrogen provides a glimpse of the future: we see a simple, square plexiglass box. The box stands alone – there are no wires attached. Then, a spotlight flashes on the cell. The camera zooms in and, lo and behold, small bubbles are rising from a screen in the middle of the cell: hydrogen from light.
In July, the researchers scored a publication about this in Nature Communications,[i] as no one had previously been able to achieve a capacity of 5% from such an integrated unit made of inexpensive materials. This is twice what nature can do: plants can store no more than 2%-3% of the solar energy that they absorb as biomass.
Abdi’s PhD supervisor, the chemist Prof. Bernard Dam (ChemE, Faculty of Applied Sciences) tells about the hydrogen cell: “We wanted to develop an inexpensive and stable cell from a photo-anode combined with a solar cell. Bismuth vanadate (BiVO4) is an inexpensive and stable oxide for the photo-anode. To date, however, it has not been very efficient. Abdi added a gradient tungsten to the cell, which increased the capacity of the photo-anode considerably.”
According to Abdi, a capacity of 10% should ultimately be feasible: “We have now reached about half of this goal. If we achieve a capacity of 10% with large-scale installations, we can bid farewell to fossil fuels.” Then we will cover our roofs with combination cells that generate hydrogen, which we will use to fill our hydrogen cars – free, and without taxes.
Combination cell
The combination cell developed by Abdi and Han is a result of the refined collaboration between the Materials for Energy Conversion and Storage (ChemE, Applied Sciences) department, headed by Prof. Bernard Dam, and the Photovoltaic Materials and Devices department (EEMCS), headed by Prof. Miro Zeman. Smets, who is currently well known for presenting the MOOC on Solar Energy, explains why. “The photo-electrochemical process of splitting water (into H+ and OH–) takes place on the surface of the bismuth vanadate. A photon releases a charge into the material, but a portion of the electric voltage running across the layer comes from the solar cell behind it. This solar cell must generate exactly the same current as the bismuth vanadate. We also need a relatively high voltage, higher than the 0.9 volt generated by a single solar cell. For this reason, we have made two PV cells that are connected in series, one behind the other. The light entering the cell is used in three stages: the blue portion of the spectrum is used in the yellow oxide layer; the green portion is used in the first solar cell, and the red portion is used in the second. The electric current and the absorption spectrums of the three components are thus precisely coordinated with each other.” To complete the story, oxygen is produced on the anode, which has a layer of cobalt-phosphorus as a catalyst. Hydrogen gas, with which the whole process started, is formed on the platinum cathode.
APPEL
The researchers had reached this point in 2013. With the funds they have received from FOM and other sources (€ 750 thousand), two new PhD students will start working on a revised model, for which the researchers have high expectations. In their proposal to NWO/FOM, the researchers even refer to the prospect of a total capacity (solar to hydrogen) of 15%.
“Now that we know how the cell works, we also know where the problems lie”, explains Dam. He is thinking of improving the charge separation (e.g. by preventing the loss of electron-hole pairs due to receding of electrons), improving the absorption of light and optimising the mobility of the charge carriers (allowing greater formation on the electrodes).
The design used by the applicants Arno Smets and Dr Wilson Smith (from Dam’s group) in their proposal, entitled APPEL,[ii] is quite different from that developed by Abdi and Han. They actually reverse the entire design: in their design, hydrogen is formed on a semi-conducting photo-cathode with a catalytic layer.
The new design involves the use of amorphous silicon carbide (SiC) as a semi-conductor material. This cathode material has the advantage of being more sensitive to the red portion of the spectrum, which makes it capable of using the last ray of sunlight to generate considerable voltage. Several obstacles must be overcome before this can happen, but the researchers are confident that, with the new design, which again uses a constellation of various Si- and SiC-based semi-conductors, they will be able to achieve a higher capacity than would be feasible by improving the existing cell configuration.
The two PhD students will continue to develop the new design in the coming years, under the supervision of Smets and Smith. For example, they will be considering ways of protecting the semi-conductor against corrosion due to water. The idea is to develop a ‘passivation layer’ that separates the semi-conductor and the water, while allowing the electrons to pass, thus producing hydrogen on the cathode.
The long run
It will also be necessary to develop new oxygen-generating catalysts to replace the usual platinum. Ideally, the anode should also continue to absorb a portion of the light. To this end, the yellowish bismuth vanadate of the cell’s surface must be replaced by a different, darker oxide, which will absorb a greater portion of the spectrum. It is hoped that this will generate a stronger current, which is expected to increase the production of hydrogen. It is obviously crucial for the solar cells in the background to be able to generate as much or more electricity with less light.
Dam acknowledges, “This is research for the long run.” Forty years ago, Japanese scientists became the first to produce hydrogen with sunlight and titanium oxide. It would be a long time, however, before the discovery of materials that could be produced both efficiently and inexpensively. This is now changing. Because such research will require more than 10 years, however, Dam would like to have research support of a more structural nature. “The production of hydrogen from sunlight is a topic that is essential to a sustainable society”, asserts Dam, who would like to see funding for applied research focus more on new industries than on existing ones.
Seven roads to solar gas
The NWO/FOM/Shell programme for CO2 neutral fuels[iii] is providing support for seven studies, selected from 32 submitted proposals. The proposal from TU Delft focuses on the production of hydrogen, while the others concern the production of fuels from CO2 and hydrogen. The funders are calling upon the researchers to look for connections between the various projects.
Prof. Koper (Leiden) is working on dyes (porphins), which have proven active in the electrochemical conversion of CO2 into methane and methanol. Dr Juurlink (Leiden) and Gleeson (FOM) aim to use vibrations to break apart CO2 molecules in order to simplify chemical reactions. Prof. Van Rooij (FOM) will be using plasma technology to convert CO2 into the more reactive CO. Prof. Gardeniers and Prof. Huskens (Twente) are developing micro-threads and membranes that will maintain the separation between hydrogen and oxygen gasses. Dr Gleeson (FOM) and Prof. Lefferts (Twente) will be using plasma technology to optimise the release of CO2 from absorption materials. Finally, Prof. De Leeuw and Dr Barosso (Utrecht) and Dr Hofmann (Eindhoven) aim to develop biologically inspired iron-sulfur catalysts that use sunlight to convert CO2 into methanol or other substances.
[ii] Earth Abundant Materials based Monolithic Photovoltaic-Photo Electrochemical Device toward 15% Solar-to-Hydrogen Conversion Efficiencies – Acronym: APPEL


Comments are closed.