Stressed bacteria protect their DNA by compacting it in a cluster. More insight into the mechanisms may lead to more effective antibiotics.
PhD researcher Natalia Vtyurina studied the mechanisms of a DNA-binding protein from starved cells called Dps. It is shaped like a ball with twelve flat surfaces and spikes protruding on the edges. The diameter is 9 nanometres, about three times the width of a DNA double helix. In case of stress, caused by starving the bacteria or exposing them to antibiotics, the DNA double helix quickly curls around these Dps balls and locks down. This mechanism helps bacteria in protecting their genome.

As a small girl in Moscow, Vtyurina was told by her father about the wonders of DNA. “Imagine, Natalia, it is like every brick of a castle has the complete blueprint incorporated. Can you believe it?”. But the biology lessons in secondary school were less than inspiring, and Natalia chose physics instead. During her study, however, she was reminded of the DNA. She then looked for opportunities to study DNA with physics. And that is how she ended up at the Kavli Institute for Bionanoscience at the TU Delft Faculty of Applied Sciences.
With fluorescent microscopy, Vtyurina was able to film the process of binding DNA molecules to Dps proteins. “It’s a very fast process,” she said. “Depending on the chemical environment, either the DNA doesn’t compact at all, or it happens within seconds as in a chain reaction, and you end up with an array of compacted DNA structure. That’s because Dps not only binds to the DNA molecules but also to other Dps molecules.” The downside of this quick process was that the molecular mechanism of Dps-DNA complex formation remained unclear.
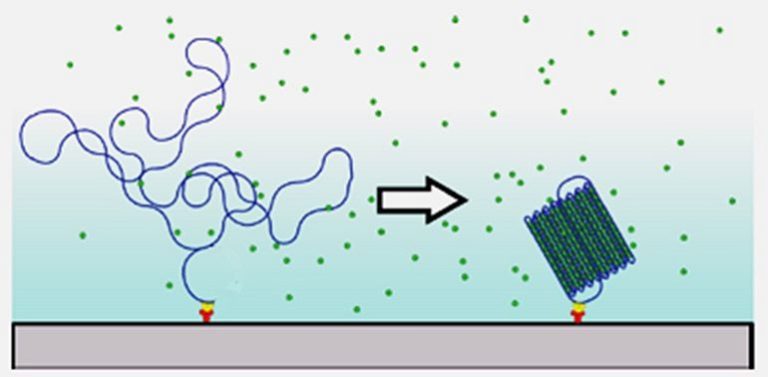
In another set of experiments at the Abbondanzieri Lab, she measured the force needed to keep a 7-micrometre piece of DNA up straight with magnetic tweezers – a powerful research tool that had been refined earlier in the department of bionanoscience. She noticed that with Dps protein present, DNA would suddenly compact below a certain force. Considerably more force was needed to undo the compacting – a phenomenon that physicists call hysteresis.
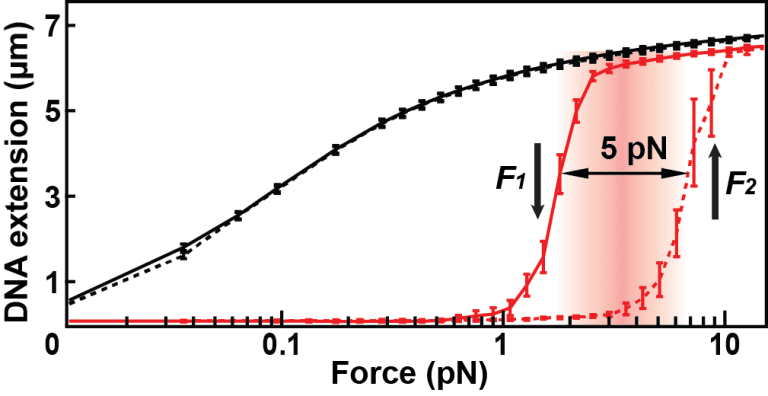
This difference in force between winding and unwinding means that bacteria under stress will quickly lock down their DNA in compact structures. However, to release the DNA, the environment must first return to favourable conditions to have the organism relax. One could say that Vtyurina measured the mechanism behind bacterial genome protection.
She also published a mathematical model describing the behaviour of the DNA-compacting in the PNAS journal (Hysteresis in DNA compaction by Dps described by an Ising model, PNAS, 2016). Her PhD supervisors Dr. Nynke Dekker and Dr. Elio Abbondanzieri co-authored the article.
Once you understand a bacteria’s defense mechanism, it should be possible to undermine it and make antibiotics more effective, right? Vtyurina agreed that, in principle, blocking Dps could make antibiotics stronger. But she stressed that a lot a fundamental science still needs to be done in this field. Additionally, studies and tests will be necessary to check the effectiveness and safety of any new drug.
–> Natalia Vtyurina, What makes long DNA short? Modulation of DNA structure by Dps protein: cooperating & reorganizing, PhD supervisors Dr. Nynke Dekker and Dr. Elio Abbondanzieri, Defence September 9, 2016, 10 AM



Comments are closed.