The Nobel Prize in Chemistry goes to the inventors of the cryo-electron microscope, a technology that has been instrumental in gaining a basic understanding of life’s chemistry and in the development of pharmaceuticals.
They captured life in atomic detail. The Nobel Prize in Chemistry 2017 is awarded to Jacques Dubochet, Joachim Frank and Richard Henderson for the development of cryo-electron microscopy, which simplifies and improves the imaging of biomolecules.
Dr Andreas Engel is happy with this decision. His group at the Department of Bionanoscience (Applied Sciences faculty) has had a cryo-electron microscope for the last half year or so. He agrees with the Nobel committee that this method has moved biochemistry into a new era.
“We have been able to look at atoms for about 30 to 40 years with electron microscopes. But up to about five years ago, looking at biological matter was not possible with this kind of microscope. You would radiate the cells to ashes. With the cryo-electron microscope, we can look at biological molecules at an atomic scale.”
Engel is especially happy for Jacques Dubochet. He knows him personally. “We worked together in the 1970s as post docs at the university of Basel. And he was here only three weeks ago at the conference that we organised on cryo-electron microscopy. As a matter of fact, I did some campaigning for him. The Nobel committee contacted me and asked if I could comment on Dubochet’s scientific merits. It is wonderful that he received this prize.”
So what exactly is the technology that Dubochet, Frank and Henderson worked on?
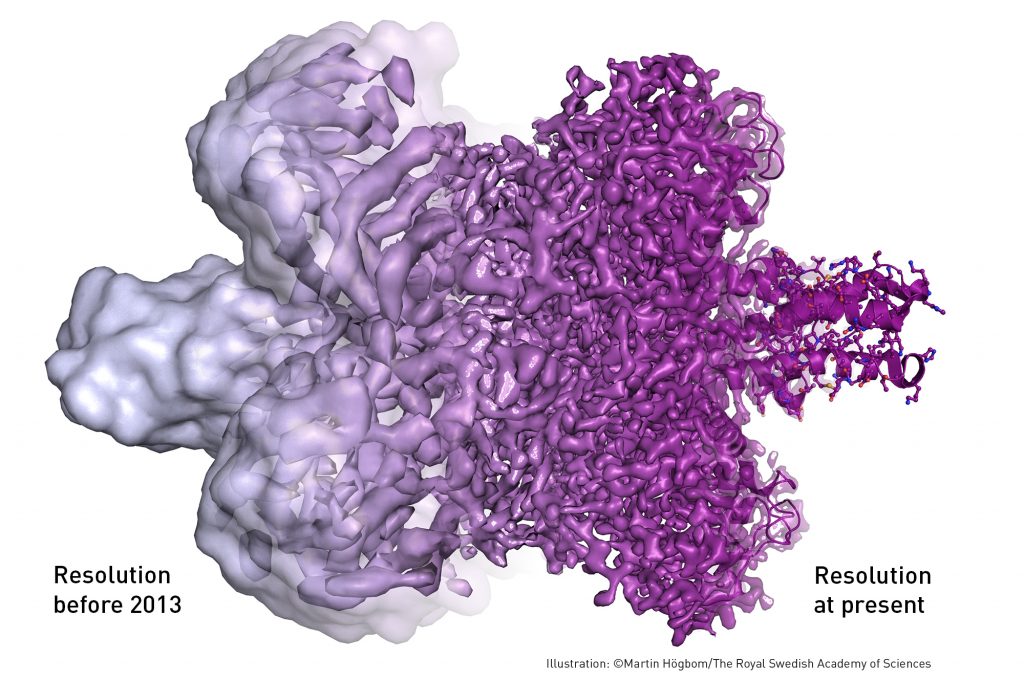
Biochemical maps have long been filled with blank spaces because the available technology has had difficulty generating images of much of life’s molecular machinery. Cryo-electron microscopy changes all of this. Researchers can now freeze biomolecules mid-movement and visualise processes they have never previously seen, which is decisive for both the basic understanding of life’s chemistry and for the development of pharmaceuticals, according to the Nobel committee.
Electron microscopes were long believed to only be suitable for imaging dead matter, because the powerful electron beam destroys biological material. But in 1990, Richard Henderson succeeded in using an electron microscope to generate a three-dimensional image of a protein at atomic resolution. This breakthrough proved the technology’s potential.
Researchers can now routinely produce three-dimensional structures of biomolecules
Joachim Frank made the technology generally applicable. Between 1975 and 1986, he developed an image processing method in which the electron microscope’s fuzzy two-dimensional images are analysed and merged to reveal a sharp three-dimensional structure.
Jacques Dubochet added water to electron microscopy. Liquid water evaporates in the electron microscope’s vacuum, which makes the biomolecules collapse. In the early 1980s, Dubochet succeeded in vitrifying water – he cooled water so rapidly that it solidified in its liquid form around a biological sample, allowing the biomolecules to retain their natural shape even in a vacuum.
Following these discoveries, the electron microscope’s every nut and bolt have been optimised. The desired atomic resolution was reached in 2013, and researchers can now routinely produce three-dimensional structures of biomolecules. In the past few years, scientific literature has been filled with images of everything from proteins that cause antibiotic resistance, to the surface of the Zika virus. Biochemistry is now facing an explosive development and is all set for an exciting future.


Comments are closed.