Scientists have discovered what causes the bright yellow hue in many of Vincent van Gogh’s paintings to fade and turn brownish over time. They’ve solved a 200 year old chemical mystery.
“Stop wasting energy! Begin an intelligent era”, roept Chintan Shah. “Make Delft the first paperless campus in the World”, schreeuwde Spark Xie. “Save energy! Save the world!”. Aan bevlogenheid hadden ze geen gebrek, de vijf TU’ers die vorige week in twee minuten tijd hun plannen voor een schonere campus over het publiek in de aula uitstortten.
De vijf waren als besten geselecteerd uit veertien inzendingen voor de Campus Energy Challenge, een initiatief van het DRI (Delft Research Initiative) Energy. De prijsvraag was een oproep om op de campus energie te besparen, duurzame energie toe te passen en de TU als proeftuin te gebruiken voor duurzame energietechnologie. Een jury van wetenschappers en deskundigen van facilitair management en vastgoedbeheer (FMVG) beoordeelde de inzendingen in twee categorieën: toepasbaar en effectief respectievelijk out-of-the-box.
De prijs voor het beste toepasbare idee ging naar de intelligent dimbare straatverlichting van ir. Chintan Shah, ir. Haibo Zhou (beiden van TBM) en ir. Vijay Rajaraman (EWI). Het beste ‘wilde idee’ was volgens de jury de inzending van student Mighael Vroom (TNW) die met restwarmte elektriciteit wil opwekken via het nog onbewezen omgekeerde magnetocalorische effect (magnetisch koelen bestaat wel). Beide winnaars ontvingen een cheque van 2.500 euro. Winnaar van de publieksprijs werd het team van Philip Hoogreef met hun Conopium windturbine.
Juryvoorzitter prof.dr.ir. Gijs van Kuik zegde toe uit te gaan zoeken hoe de dimbare straatverlichting op de campus gerealiseerd kan worden.
The scientists, among them paint expert, Professor Joris Dik (faculty MMME), took samples of yellow paint that had turned brown from the paintings ‘View of Arles with Irises’ (1888) and ‘Bank of the Seine’ (1887), both on display in the Van Gogh Museum in Amsterdam. Through X-ray analysis they discovered that the darkening of the top layer was caused by a reduction of the chromium in the chrome yellow (from Cr(VI) to Cr(III)). Prof. Dik and his colleagues published the results this week online in the journal Analytical Chemistry.
The research, led by Koen Janssens of Antwerp University, was further conducted by an international team of scientists from four countries. The scientists deployed an impressive arsenal of analytical tools, with synchrotron X-rays at the European Synchrotron Radiation Facility (ESRF) in Grenoble (France) providing the final answers. “I am not aware of a similarly big effort ever having been made for the chemistry of an oil painting,” said Prof. Dik in an ESRF press release.
According to the professor, the faded paint also contained lots of barium and sulphur. “These atoms probably originate from barium sulphate, a colourless filling material that was added to the paint. This filling might be the cause of the change in colour.”
The researchers also collected samples from three left-over historic paint tubes. After these samples had been artificially aged for 500 hours using an UV-lamp, only one sample, from a paint tube belonging to the Flemish Fauvist Rik Wouters (1882-1913), showed significant darkening. Within three weeks its surface of originally bright yellow had become chocolate brown. This sample was taken as the best candidate for having undergone the fatal chemical reaction, and X-ray analysis identified the darkening of the top layer as linked to a reduction of the chromium.
This is not the first time Prof. Dik and his colleagues have made headlines with their research on paintings. In 2008, using X-ray techniques as well, they discovered an early rejected painting of a face of a woman underneath Van Gogh’s ‘Patch of Grass’ painting.
Do you have a question or comment about this article?
tomas.vandijk@tudelft.nl

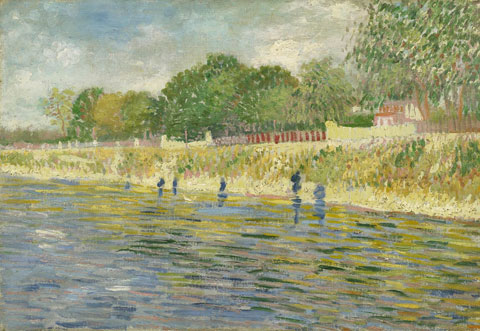
Comments are closed.