Scientists from TU Delft and AMOLF engineered an efficient and stable photoelectrode, a material that absorbs light and directly splits water into hydrogen and oxygen.
Moses parting the Red Sea (Image: Paramount Pictures)
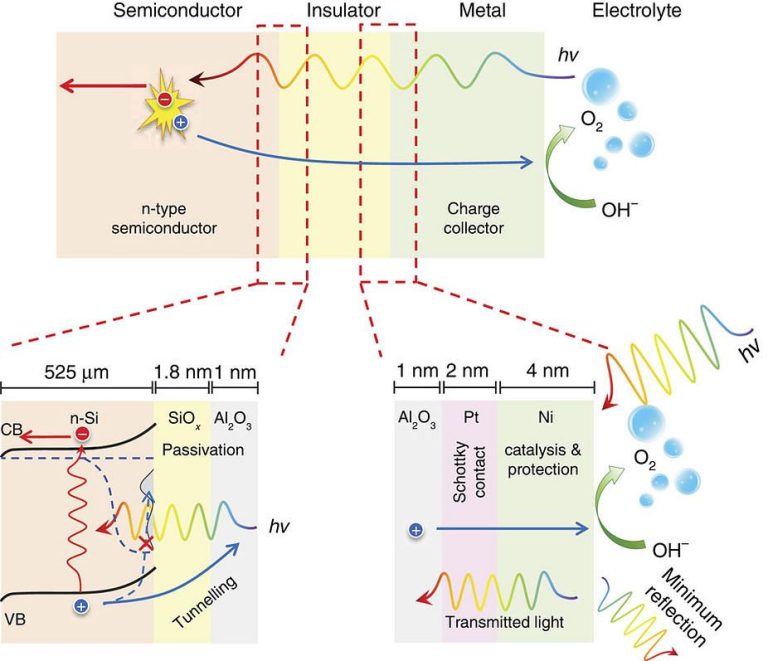
 Schematic of the photoanodes for water oxidation, showing functionalities of each layer
Schematic of the photoanodes for water oxidation, showing functionalities of each layerPhotoelectrochemical water splitting (into hydrogen and oxygen) is a promising method of producing clean and renewable fuel from the direct conversion of solar to chemical energy.
Sunlight excites free electrons near the surface of the silicon electrode. These electrons flow through wires to the stainless steel electrode, where they react with water molecules to form hydrogen and four OH groups.
Photo-electrode with a service life of 200 hours
However, corrosion of the semiconductors remains an issue, given their direct contact with water. This limits the service life of photoelectrochemical cells. Together with colleagues from AMOLF, Delft scientists engineered a photo-electrode, with a service life of 200 hours.
This is an impressive feat. Up to now, the kind of photoelectrochemical cells that the team has worked on only yielded a few hours of stability.
That being said, there is still a long way to go. Research is now underway to reach a service life of 10,000 hours, a requirement set by the United States Department of Energy.
The researchers use silicon wafers as the light absorbing material, so the system is also cheap. They reported their findings in Nature Communications on Thursday June 29th.
“In summary, we now have a material that is low cost, absorbs a lot of light, has a high catalytic efficiency, and is remarkably stable,” says Wilson Smith, leader of the team and Associate Professor in the Department of Chemical Engineering at TU Delft in a press release.
Newly designed insulator layer
It is essential for a photoelectrochemical system to provide a sufficiently high photocurrent and photovoltage to drive the water oxidation reaction. Typically, there is a balance between the catalytic efficiency of this system and its long-term stability. Fixing one problem usually makes the other worse.
“Here, we have independently addressed the stability and catalysis bottlenecks in photoelectrochemical water splitting, and combined them into one simple system,” says Smith. “We used a newly designed insulator layer to stabilise the semiconductor photoelectrode, while also using two metals to increase photovoltage and split water with a high efficiency.”
Ibadillah Digdaya, Gede Adhyaksa, Bartek Trześniewski, Erik Garnett and Wilson Smith, ‘Interfacial engineering of metal-insulator-semiconductor junctions for efficient and stable photoelectrochemical water oxidation’, DOI: http://dx.doi.org/10.1038/NCOMMS15968
 Schematic of the photoanodes for water oxidation, showing functionalities of each layer
Schematic of the photoanodes for water oxidation, showing functionalities of each layerDo you have a question or comment about this article?
tomas.vandijk@tudelft.nl


Comments are closed.