A protein involved in folding and clamping DNA in every living creature has for the first time been shown in action. Researchers from Delft and Heidelberg demonstrated that the protein condensin is much more dynamic than assumed.
DNA molecules are like extremely long strands of fairy lights: it’s almost impossible to disentangle them and store them neatly. A single human cell contains two metres of DNA molecules but is somehow able to fold them neatly into chromosomes measuring just a few micrometres. The protein condensin is known to play a crucial role, but the details of the process are still largely a mystery. For the first time, researchers from TU Delft’s Kavli Institute of Nanoscience and the European Molecular Biology Laboratory in Heidelberg have successfully filmed the behaviour of an individual condensin molecule.
“Until now, condensin was seen as a somewhat stiff molecule. To try to explain it, you can compare it with a hair clip: condensin secured the DNA in loops by clicking open and closed. But our latest research shows that it would be better to compare it with an elastic band,” said Jorine Eeftens, a doctoral candidate in Cees Dekker’s research group. She worked with postdoc Allard Katan on the microscopy. “We witnessed condensin as an extremely dynamic molecule, darting back and forth before our eyes under the microscope. We also discovered that condensin has four different positions rather than two: not just open and closed but also a sort of double loop and a semi-open position Condensin probably uses these different positions to attach to a DNA strand before storing it away compactly. We are now conducting further experiments to find out exactly how this works.”
Earlier studies on the so-called Structural Maintenance of Chromosomes (SMC) protein complexes were done with electron microscopy or with X-ray scattering. Although the imaging is very precise, these techniques need the protein under study to be either dried or packed into a crystal lattice prohibiting any natural occurring dynamics.
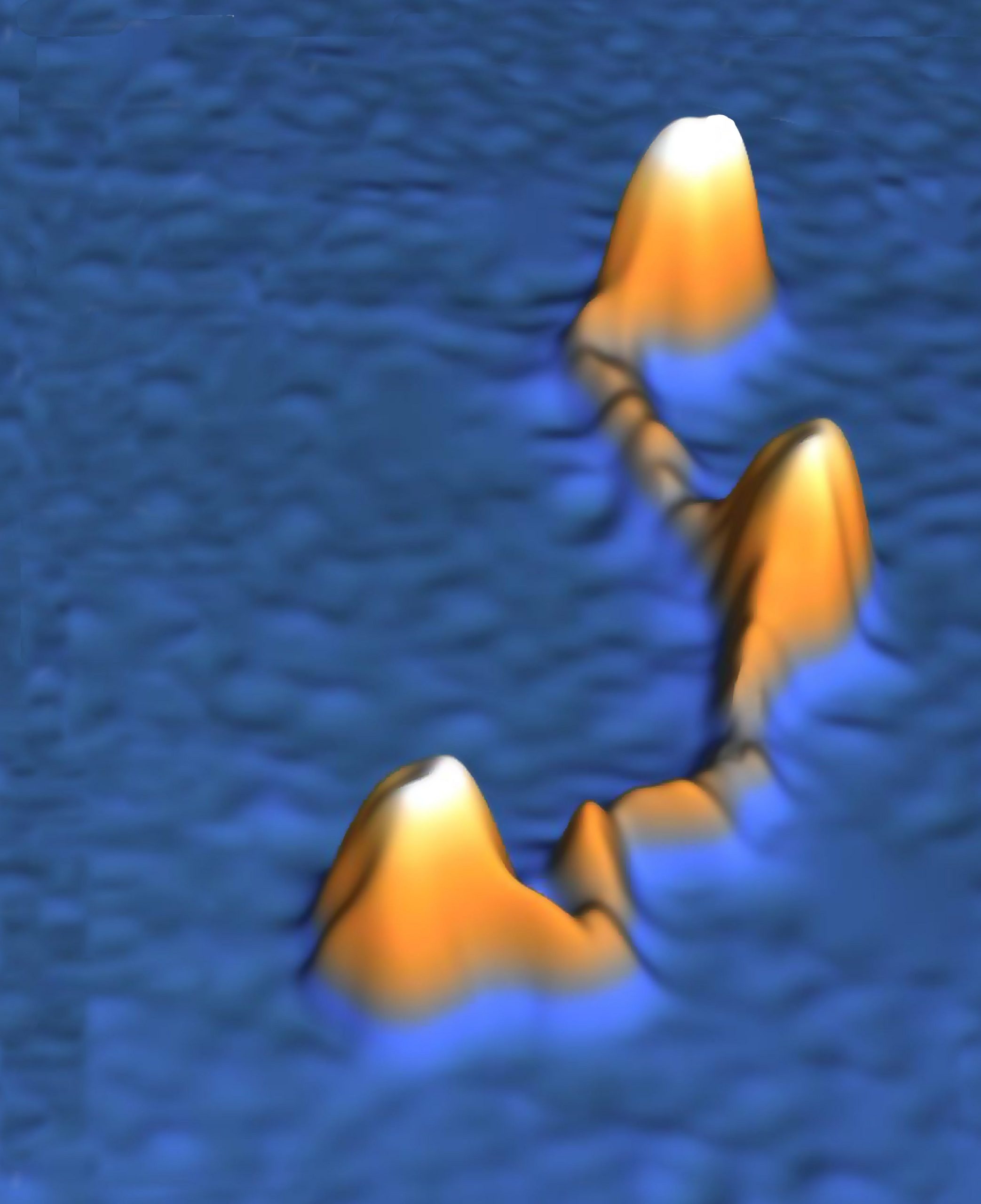

This, however, was the first time that the proteins were studied in a wet physiological environment with atomic force microscopy (AFM). This technique allowed the researchers to study the protein complexes in their natural environment. High-speed AFM even allows recording movies with a frame rate of 10 per second. As Eeftens so vividly described, the protein behaved much more flexible and dynamic than expected. In their own words: ‘We conclude that the coiled coils are not stiff rods, but are instead highly flexible.’
The researchers, led by Professors Christian Haering from Heidelberg and Cees Dekker from Delft, conclude that their study has shown the basics underlying the DNA-entrapment by this protein complexe. It also sets the stage for further in-depth studies of the proteins involved.
Jorine M. Eeftens, Allard J. Katan et. al., Condensin Smc2-Smc4 dimers are flexible and dynamic, Cell Reports, February 18, 2016.



Comments are closed.